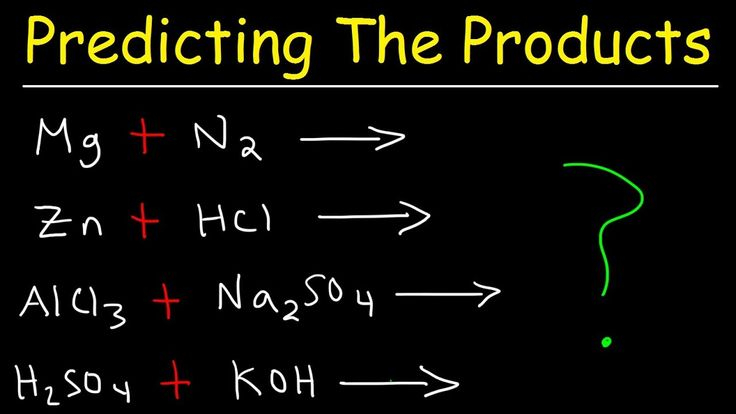
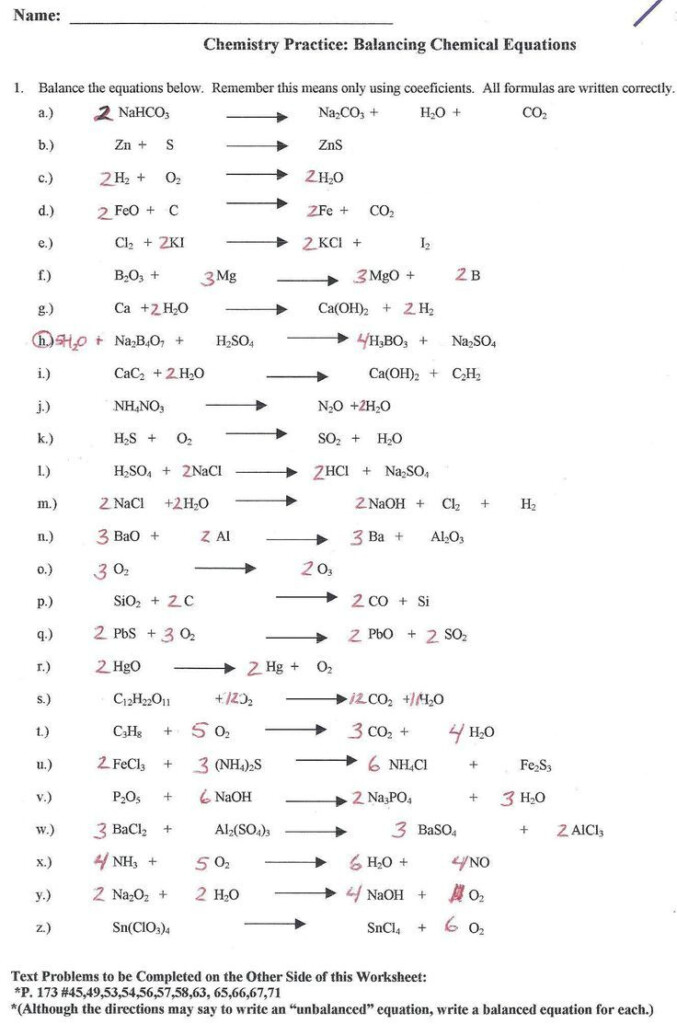
Chemistry Predicting Reactions Worksheet – The Chemistry Reactions Worksheet can be used to help students understand the concepts of chemical changes. A chemical reaction involves the transfer of energy between reactants and products. This type of change can be reversible or irreversible. This happens when two molecules or atoms react to create a new product.
Changes in the bond structure can cause chemical reactions
Chemical reactions are the process of creating new molecules by breaking or forming bonds between substances. These reactions require energy because it takes energy to break bonds, and then release the product. Different types of bond structure produce different amounts of energy. A Lewis acid-base reaction, for example, produces a covalent bonds, in which the Lewis acid provides an electron pair, and the Lewis base receives one.
The energy involved in chemical reactions can be approximated using the bond strengths of reactants and products. These bond strengths change as a result of the chemical reactions. This energy can be measured in terms of heat, enthalpy and thermal energy. The energy of chemical reactions is also expressed at the atomic level as potential energy. This idea of energy is not often explained in chemistry textbooks.
These involve energy transfer between products and reactants.
In chemical reactions, energy is transferred from reactants to products. The energy is transferred through the form of bonds. Bond energy, also known as bond energy, is measured in kJ*mol$-1. The energy of the products and reactants determines how much energy can be transferred.
Understanding chemical reactions is key to understanding how energy is transferred. These reactions are known as energy change. This is energy absorption or release that occurs when chemical bonds break. Generally, this energy is a form of heat or light, depending on the reactants and products. The energy transfer occurs because of the differences in stored chemical energy, or enthalpy.
They can be reversed
When both products and reactants are converted to one another in a chemical reaction, the process is known as a reversible reaction. It occurs when the conversion of the reactants to the products occurs simultaneously. This reaction is the most common in chemistry. Here’s how it works.
A reaction that occurs between a substance and a gas can be reversible or irreversible. A product is when an acid reacts to an alcohol. To allow this reaction to take place, it is necessary to let go of any gas molecules that were previously bound with the solution. The Dean-Stark apparatus separates the reactants and ensures that the desired product can be produced.
They cannot be reversed.
There are several kinds of reactions in chemistry. The type of reaction will depend on the reactants and surroundings. The majority of chemical reactions can’t be reversed. They involve the conversion of two or more reactants into one or more products. Sometimes the catalyst can be used to enhance the reaction.
A reversible reaction is one that occurs in a closed container. Ammonium chloride, for example, can be heated to make ammonia or hydrogen chloride. It is then converted to ammonium chloride when it cools. These two reactants will then recombine.
They involve redox reactions
Redox reactions involve the transfer of electrons between different chemical species. The oxidation process results in the loss of one or several electrons by an oxidizing agent, while the reduction process results in the gain of electrons from the reducing agent. Redox reactions can have a wide range of effects on environmental variables such as contaminant mobility or degradation. Hexavalent chromium, for example, is extremely toxic when it is oxidized. Trivalent chromium, on the other hand, is less toxic and less mobile. Arsenic and uranium are also less mobile in oxidizing conditions.
Redox reactions can also occur during decomposition processes. This results in a smaller chemical compound. CaCO3 will react with CO2 to form COO, but the oxidizing agent will gain an electron. An oxidizing agent can also gain oxygen and bring it into the molecule. Typical oxidative reactions in organic chemistry include dealkylation, epoxidation, aromatic ring cleavage, and hydroxylation.
They involve acids and bases
A Chemistry reaction is when acids and bases react with one another to create a new substance. A salt is a substance that forms when an acid reacts with a base. Salts are crystalline substances that are soluble in water. They can also be bitter. There are many theories as to how acids and bases interact with one another.
Both acids and bases play important roles in chemical reactions and in daily life. For example, the presence of acid in the body helps keep the internal environment stable. Acidity is also important in baking cakes. The acidity of a lake determines whether it can sustain aquatic life. As a result, a large percentage of chemical processes involve either acids or bases. Acids and bases also play a key role in biological processes. The pH and alkalinity in the soil and water are crucial for animals and plants. The chemistry of acids or bases is a constant part of our everyday lives.