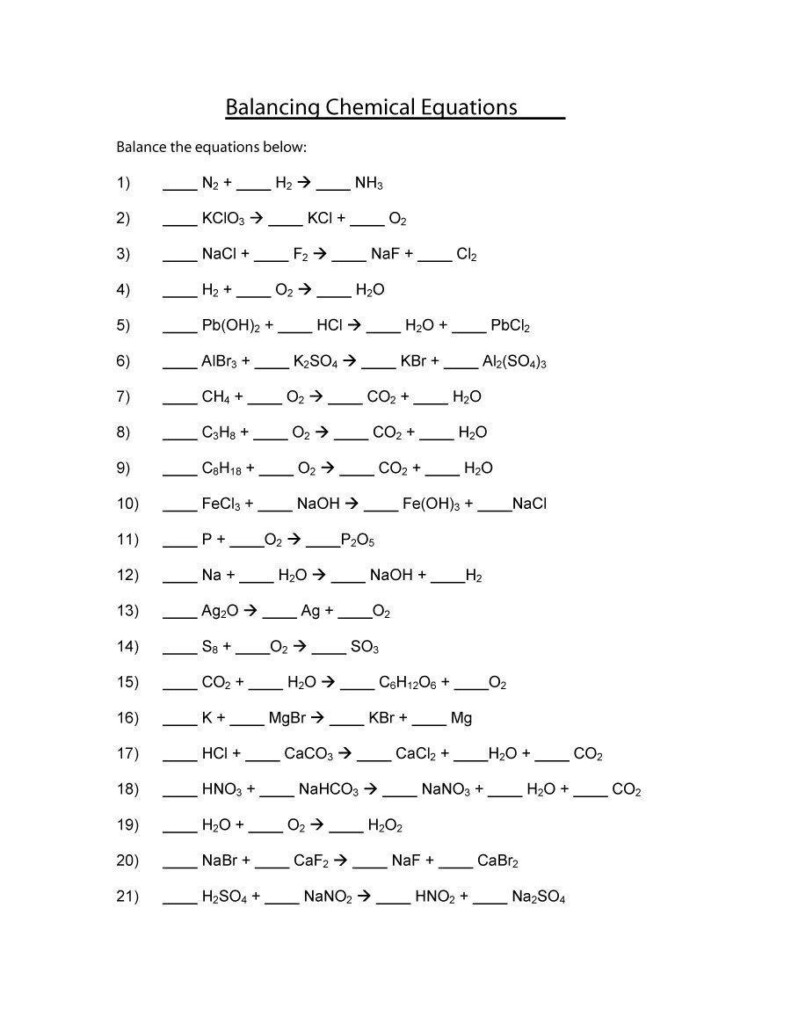
Chemistry Balancing Equations Worksheet 2 – This Chemistry Equations Worksheet will help students understand the language of chemical equations. This worksheet should be completed after students have learned the rules of chemical formulae, bonding and state symbols, and enables teachers to provide feedback on where students may be lacking. The worksheet is divided into two parts. Page one summarizes the rules for writing chemical equations. Some of these rules may not be applicable to A Level students.
Chemical equations in balance
A chemical equation has two parts: a reactant and a product. An arrow separates the equation. In the example below, H2 and O2 are the reactants and H20 and CO are the products. Balance a chemical equation requires that the products and reactants must be equal. This can be difficult, but there are some tricks to balance a chemical equation.
To help students learn how to balance chemical equations, there is a balancing chemical equations worksheet available online. The worksheet includes ten unbalanced equations as well as an answer key. It is a great resource for elementary students learning about the chemical process. A student can complete the worksheet on their tablet and check their understanding with the answer key provided.
A balancing Chemical Equations worksheet is a visual activity that helps students to understand the differences between coefficients and subscripts when they are learning about balancing. This worksheet explains why balance chemical equations is so important and how it can be done. Using a balancing chemical equations worksheet makes it easy to create multiple worksheets, with each worksheet containing a matching answer sheet.
When you are learning about chemical equations, you have to understand the Law of Conservation of Mass. The Law of Conservation of Mass says that both sides of a chemical equation must have equal numbers of atoms. To balance a chemical equation, first find an element that has a single reactant and one product. Then, use this element to balance the first one.
Classify reactions
A chemistry equation describes a chemical reaction in mathematical terms. For example, magnesium reacts with oxygen gas to form a solid magnesium oxide, or calcium metal reacts with water to form calcium hydroxide precipitate. Ammonia is formed when nitrogen reacts with hydrogen gas to form gaseous ammonia. However, unlike many other chemical reactions, ammonia is not destroyed during the reaction, and so the atoms remain unchanged.
Chemical reactions are a common part of everyday life. These processes change the chemical structure of reactants and produce new substances. The changes in these reactions occur because they change the bond structures of the substances. The changes can involve energy use or release, and many physical indicators can be observed. These characteristics are used to classify reactions.
The common chemical reaction acid-base is found in nature. Both reactants exchange electrons. When an acid reacts with a base, it neutralizes the acid. An acid and a base can also react to each other to create a new substance. Similarly, a precipitation reaction is a reaction in which the reactants disperse in a liquid. Depending on the solubility properties of the reactants, different precipitates can be formed. Redox reactions involve the transfer electrons between several reactants, and can result in ionic compounds. In addition, hydrolysis reactions use water as one of the reactants. They produce smaller products such as CO and H2.
Calculate coefficients
To solve chemical equations, the first step is to find the coefficients. The coefficients are whole numbers that represent the amount of each element in a chemical equation. These coefficients are calculated by balancing both the numbers of the elements on each side of an equation. For example, if a chemical has 10 atoms of oxygen and one atom of sulfur, the coefficient for oxygen would be 2. If the atoms on the opposite side of the equation have the same number, they are said to be a mixed compound.
You must correctly write reactants and products in order to balance chemical equations. In addition, you should also indicate the state of the substances with the help of symbols. If the substance is a salt, for example, it should be written NaCl. It cannot be written as Na2Cl2.
The next step in solving chemistry equations is to find the balance between the atoms and molecules. As we all know, the ratio of two substances in a chemical equation must be equal on both sides. In addition, chemical equations must be balanced because the atoms must have the same number of each type of atom.
To determine the molar mass for a given element, you can use stoichiometric relationships to solve a chemical problem. By calculating the molar mass, you can convert Fe(s) into H2(g). For example, the molar mass of H2O is two times that of a single H atom.