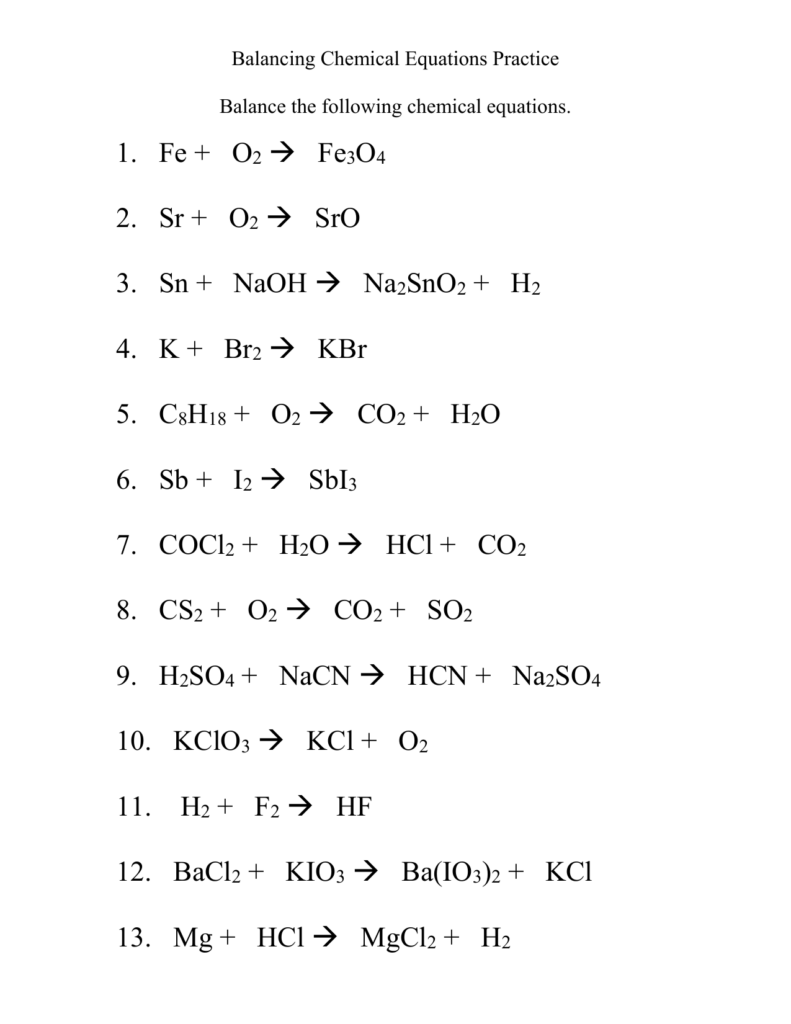
Chemistry 1 Balancing Equations Worksheet – This Chemistry Equations Worksheet will help students understand the language of chemical equations. This worksheet is meant to be completed once students have learned the rules for chemical formulae, bonding, and state symbols. It also allows teachers to give feedback and suggestions on areas students might be struggling with. This worksheet consists of two parts: page one summarizes the rules of writing chemical equations. However, some of these rules will not be relevant for A Level students.
Balance chemical equations
A chemical equation has two parts: a reactant and a product. The equation is separated by an arrow, so in the example below, the reactants are H2 and O2 and the products are H20 and CO2. Balance a chemical equation requires that the products and reactants must be equal. It can be hard to balance a chemical formula. However, there are ways around this.
A balancing chemical equations worksheet is available online to help students balance chemical equations. This worksheet contains ten unbalanced equations and an answer key. It is a great resource for elementary students learning about the chemical process. The worksheet can be completed on a tablet by students. They can also check their understanding using the answer key.
A balancing Chemical Equations worksheet is a visual activity that helps students to understand the differences between coefficients and subscripts when they are learning about balancing. This worksheet explains why balance chemical equations is so important and how it can be done. A balancing chemical equations worksheet allows you to easily create multiple worksheets. Each worksheet contains a matching answer sheet.
When you are learning about chemical equations, you have to understand the Law of Conservation of Mass. The Law of Conservation of Mass states that the amount of atoms on both sides of a chemical equation should be the same. To balance a chemical equation, first find an element that has a single reactant and one product. Then, use this element to balance the first one.
Classify reactions
A chemistry equation is a mathematical formula that describes a chemical reaction. For example, magnesium reacts with oxygen gas to form a solid magnesium oxide, or calcium metal reacts with water to form calcium hydroxide precipitate. When nitrogen reacts with hydrogen gas, ammonia is formed. The reaction does not destroy ammonia, which is unlike other chemical reactions. Therefore, the atoms are unaltered.
Chemical reactions are common everyday processes. These reactions alter the chemical structure of reactants, and create new substances. These reactions cause changes in the bonds structures of substances. These changes can occur as a result of energy use or release. Many physical indicators can also be observed. These characteristics help classify reactions.
An acid-base reaction is a common chemical reaction in nature. Both reactants exchange electrons. An acid reacts with a basic substance to neutralize it. An acid and a base can also react to each other to create a new substance. Similarly, a precipitation reaction is a reaction in which the reactants disperse in a liquid. Different precipitates may be formed depending on the solubility of the reactants. There are also redox reactions, which involve the transfer of electrons between two or more reactants and result in ionic products. Hydrolysis reactions also use water as one reactant. They produce smaller products like CO2 and H2.
Determine coefficients
To solve chemical equations, the first step is to find the coefficients. The coefficients are whole numbers that represent the amount of each element in a chemical equation. These coefficients are calculated by balancing both the numbers of the elements on each side of an equation. For example, if a chemical has 10 atoms of oxygen and one atom of sulfur, the coefficient for oxygen would be 2. A mixed compound is one in which the atoms on opposite sides of the equation share the same number.
To balance chemical equations, you should write the reactants and products correctly. In addition, you should also indicate the state of the substances with the help of symbols. If the substance is a salt, for example, it should be written NaCl. It cannot be written as Na2Cl2.
Next, we need to balance the atoms with the molecules in order to solve chemistry equations. As we all know, the ratio of two substances in a chemical equation must be equal on both sides. In addition, chemical equations must be balanced because the atoms must have the same number of each type of atom.
Using stoichiometric relations to solve a chemical equation, you can determine the molar mass of a given element. Calculating the molar mass will allow you to convert Fe(s), into H2(g). For example, the molar mass of H2O is two times that of a single H atom.