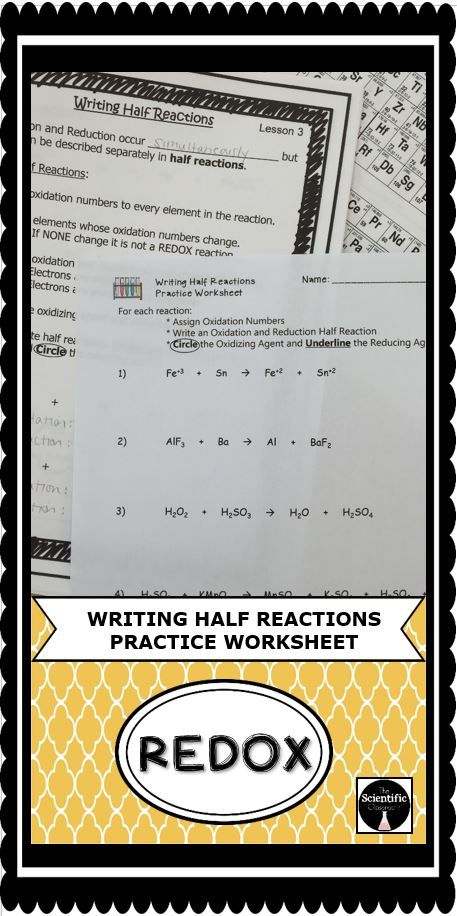
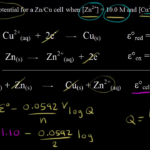Ap Chemistry Balancing Redox Reactions Worksheet – The Chemistry Reactions Worksheet can be used to help students understand the concepts of chemical changes. A chemical reaction involves the transfer of energy between reactants and products. This type of change is either irreversible or reversible. This happens when two molecules or atoms react to create a new product.
Changes in the bond structure can cause chemical reactions
Chemical reactions are the process of creating new molecules by breaking or forming bonds between substances. These reactions are energy-intensive because energy is required to break bonds and then be released in a product. Different types of bond structures produce different amounts energy. For example, a Lewis acid-base reaction produces a covalent bond, where the Lewis acid supplies an electron pair and the Lewis base accepts one.
The energy involved in chemical reactions can be approximated using the bond strengths of reactants and products. The chemical reactions can cause these bond strengths to change. This energy is measured in terms of enthalpy, heat, and thermal energy. The energy of chemical reactions is also expressed at the atomic level as potential energy. This idea of energy is not often explained in chemistry textbooks.
They involve the transfer of energy between reactants and products
Chemical reactions involve energy being transferred from reactants into products. The energy is transferred through the form of bonds. Bond energy, also known as bond energy, is measured in kJ*mol$-1. The energy of the products and reactants determines how much energy can be transferred.
To understand how energy is transferred, we must first understand how chemical reactions occur. These reactions are known as energy change. This is energy absorption or release that occurs when chemical bonds break. Generally, this energy is a form of heat or light, depending on the reactants and products. Energy transfer is caused by the difference in chemical energy stored, also known as enthalpy.
They are reversible
Reversible reactions are when both reactants and products are converted to each other in a chemical reaction. This happens when both reactants and products are converted simultaneously. This is one of the most common reactions in chemistry. This is how it works.
Reversible reactions between substances and gases can either be irreversible or reversible. A product is when an acid reacts to an alcohol. In order for this reaction to occur, the gas molecules that were previously bound to the solution must be released. A Dean-Stark apparatus is used to separate the reactants, ensuring that the desired product is produced.
They cannot be reversed.
There are several kinds of reactions in chemistry. Reactants and their surroundings will determine the type of reaction. Most chemical reactions are irreversible. They involve the conversion of two or more reactants into one or more products. Sometimes the catalyst can be used to enhance the reaction.
A reversible reaction is one that occurs in a closed container. For example, ammonium chloride can turn into ammonia and hydrogen chloride when heated. When it cools, it is converted back to ammonium chloride. The two reactants then recombine.
They involve redox reactions
Redox reactions involve the transfer of electrons between different chemical species. The oxidation process results in the loss of one or several electrons by an oxidizing agent, while the reduction process results in the gain of electrons from the reducing agent. Redox reactions can have a wide range of effects on environmental variables such as contaminant mobility or degradation. Hexavalent chromium, for example, is extremely toxic when it is oxidized. Trivalent chromium, on the other hand, is less toxic and less mobile. Arsenic and uranium are also less mobile in oxidizing conditions.
During decomposition, redox reactions may also occur. This results in a smaller chemical compound. CaCO3 will react with CO2 to form COO, but the oxidizing agent will gain an electron. An oxidizing agent can also gain oxygen and bring it into the molecule. The most common oxidative reactions in organic Chemistry include dealkylation and aromatic ring cleavage.
They involve acids and bases
A Chemistry reaction involves acids and bases reacting with each other to produce a new substance. When the acid reacts with the base, it produces a new substance called a salt. Salts are crystal substances that dissolve in water. They can also be bitter. There are many theories about the way acid and bases react with each other.
Both acids and bases play important roles in chemical reactions and in daily life. For example, the presence of acid in the body helps keep the internal environment stable. Acidity is also important in baking cakes. The acidity of a lake determines whether it can sustain aquatic life. A large number of chemical reactions involve either acids and bases. Both acids and bases play an important role in biological processes. The pH and alkalinity in the soil and water are crucial for animals and plants. As such, the chemistry of acids and bases is ubiquitous and permeates our daily lives.